Introduction
Alzheimer’s disease is a debilitating neurodegenerative condition, marked by progressive memory loss, cognitive decline, and the presence of beta-amyloid plaques and tau tangles in the brain. Despite decades of research, no definitive cure exists.
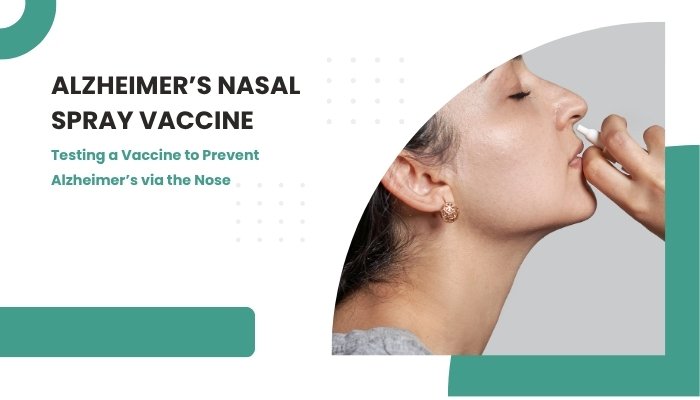
However, a novel strategy is generating excitement: a nasal spray vaccine designed to stimulate the immune system into preventing or slowing down Alzheimer’s pathology. Though still in early-stage trials, this approach could revolutionize how we combat one of the world’s most pressing age-related conditions.
This article explores how this nasal vaccine might work, the science behind it, and what challenges remain on the road to making this potential therapy a reality.
Why a Vaccine for Alzheimer’s?
Immune System and Brain Health
For years, Alzheimer’s research has grappled with removing or halting the accumulation of beta-amyloid, a sticky protein fragment forming plaques in the brain, and tau, a protein that aggregates into neurofibrillary tangles. While numerous experimental antibodies have aimed to clear these proteins, results have been mixed, often with side effects like brain swelling.
The idea of a “vaccine” harnesses a preventive immunologic response—not just a passive antibody infusion—to teach the body how to detect and neutralize harmful proteins early. With Alzheimer’s onset typically occurring over decades, a vaccine might intervene in the slow buildup of amyloid or tau, potentially delaying or preventing disease progression.
The Nasal Delivery Advantage
Nasal sprays offer a direct route to immune-related tissues in the nasal passages, potentially stimulating both systemic and mucosal immunity. For central nervous system (CNS) diseases, this route can more readily influence brain-related immune cells without the typical challenges that come with injections or intravenous drugs.
How the Nasal Spray Vaccine Works
Targeting Beta-Amyloid
The vaccine under development typically includes compounds that:
- Promote an Immune Response: Elicit the production of antibodies or immune cells specifically recognizing beta-amyloid (or possibly tau).
- Clear or Prevent Plaques: By binding to these proteins, the immune system can mark them for clearance, reducing plaque formation or slowing further buildup.
Stimulating Key Immune Pathways
Researchers often use adjuvants—substances that boost immunogenicity—to ensure the body strongly responds to the vaccine. By spraying the formulation into the nose:
- Local Immune Cells Activation: Nasal-associated lymphoid tissue can quickly alert and mobilize broader immune pathways.
- Crossing into the Brain: Some immune cells or factors that traverse from nasal passages may help remove amyloid from the brain over time, or prevent it from clumping.
Current Status of Clinical Trials
Early-Phase Trials
A few institutions, including leading academic hospitals, have launched Phase I or Phase I/II trials with small groups of volunteers—often older adults at risk or showing early cognitive changes. Researchers track:
- Safety and Tolerability: Ensuring no excessive inflammation, nasal irritation, or severe side effects.
- Immune Response: Measuring whether participants develop amyloid-targeting antibodies or T-cell responses.
- Early Cognitive Measures: While short trials may not confirm long-term prevention, subtle changes in biomarkers or mental function are monitored.
Preliminary Findings
Though data is limited, initial results appear promising:
- Mild Side Effects: Some participants report slight nasal discomfort or mild headaches, but no major systemic reactions.
- Immune Activation: Indications of boosted anti-amyloid or anti-tau immunity.
- Future Phases: Larger trials will assess efficacy—whether the vaccine truly slows or halts cognitive decline in moderate at-risk populations.
Potential Benefits and Impact
- Early Intervention: A vaccine approach targets the disease at or before symptom onset, potentially staving off significant decline.
- Less Invasive: Nasal sprays are user-friendly—compared to IV infusions or frequent injections—improving patient adherence.
- Reduced Healthcare Burden: If effective, widespread vaccination for at-risk groups (e.g., older adults) might save healthcare systems billions in advanced dementia care.
Challenges and Unknowns
Need for Prolonged Data
Alzheimer’s can progress over decades, so short clinical trials (6–12 months) might not show the full preventive effect. Multi-year studies are crucial to see if cognitive improvements persist or if memory decline is delayed significantly.
Heterogeneity of Alzheimer’s
Alzheimer’s is complex—some patients have more tau pathology, others might have vascular components or additional neurodegenerative processes. A single vaccine focusing on amyloid or tau might not help all cases.
Regulatory and Ethical Considerations
Launching a wide vaccination program for aging populations requires robust evidence, cost-effectiveness analysis, and ethical frameworks—especially if vaccinating asymptomatic individuals.
Frequently Asked Questions
- Is the nasal vaccine guaranteed to prevent Alzheimer’s?
- Too early to say. It’s experimental. While initial results are encouraging, more extensive and long-term research is needed.
- Could children or younger adults get this vaccine proactively?
- Not anytime soon. Trials currently focus on older adults at risk or with mild impairment. The concept of universal adult vaccination is hypothetical until safety and efficacy are proven.
- How does it differ from other Alzheimer’s treatments?
- Many existing therapies treat symptoms or slow progression. A vaccine aims at prophylaxis or early disease modification by mobilizing the immune system over the long term.
- Are there side effects like severe inflammation or autoimmune reactions?
- Early data suggests mild local nasal reactions or headaches. Larger trials will clarify the risk of more serious issues, including potential neuroinflammation.
- When might it be available to the public?
- If advanced Phase III trials confirm efficacy and minimal side effects, approval could come in several years. The timeframe depends on trial success and regulatory review speed.
Conclusion
A nasal spray vaccine for Alzheimer’s disease marks a novel frontier in tackling one of the most pervasive neurodegenerative conditions. By stimulating an immune response specifically against amyloid or tau proteins, researchers hope to either delay or entirely stave off cognitive impairment. Though far from mainstream adoption, the initial results from early-phase trials inspire cautious optimism. Should ongoing and future studies replicate these findings in larger, more diverse cohorts—and confirm a robust safety profile—this approach could become a major tool for reducing Alzheimer’s incidence. In the meantime, the quest for an effective, preventive therapy continues, fueled by the promise that a simple nasal spray might shield millions from the debilitating grip of dementia.
References
-
- Brigham and Women’s Hospital. (2022). “Nasal vaccine development for Alzheimer’s.”
-
- Alzheimer’s Association. (2023). “Experimental therapies in early-stage trials.”
-
- NIH. (2023). “Neuroimmunological strategies for AD prevention.”
-
- Nature Neuroscience. (2021). “Immunization approaches targeting amyloid-beta in AD.”